Kirchoff's Laws of Spectroscopy
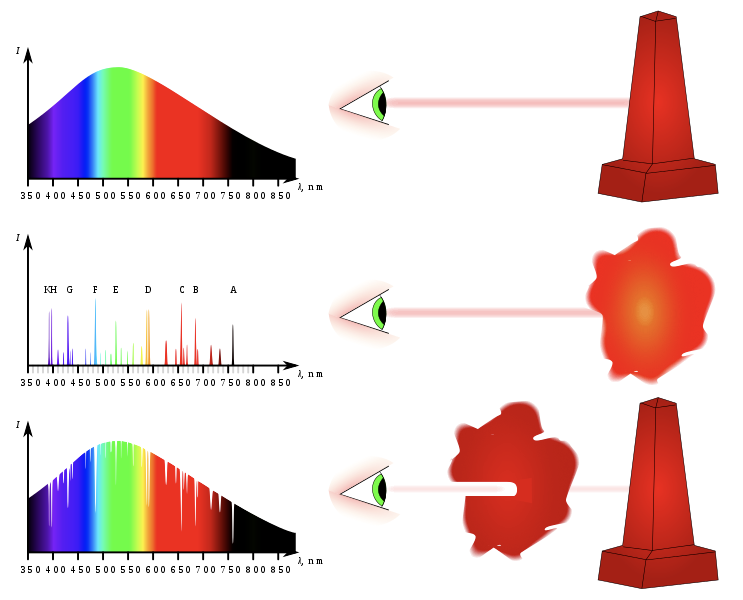
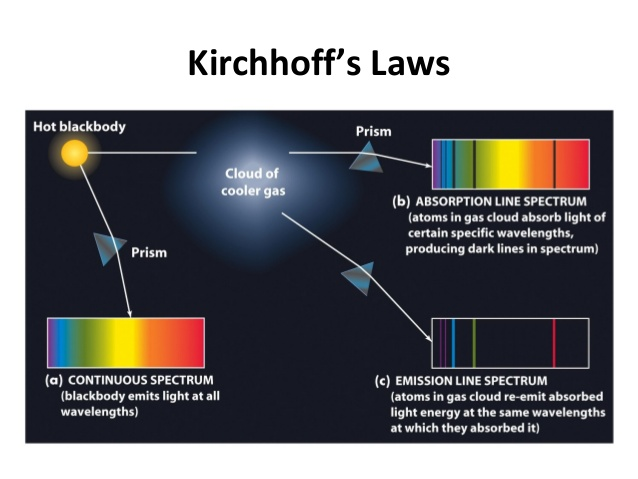
- Every element has a finger print
- They interact with light in their own way
- Either in absorption or emission, finger print or spectral lines are in the same place
- A luminous solid, liquid, or dense gas emits light of all wavelengths
- A low density, hot gas seen against a cooler background emits an emission spectrum
- Looks all black, except for light being emitted
- A low density, cool gas in front of a hotter source of a continuous spectrum creates an absorption spectrum
Spectroscopy Physics

